

Jacob Øhrgaard
CSO – Chief Sales Officer
Dear Customers & Distributors
We hope that you are all well and safe. Fall is just around the corner and we at Vitrex have some important news we would like to share with you.
For all of us working with Medical devices there is a serious change ongoing in the regulatory landscape. This change has a significant impact on all manufacturers, importers and distributors of medical devices, in terms of added need to invest resources, time and money in transferring from the Medical device directives MDD/IVDD to the new Medical device regulation MDR/IVDR. The new Medical device regulation has imposed new product classification, EUDAMED changes and increased requirements for the Clinical Evaluation process, Post-Market Surveillance, Quality Management systems. This with the purpose of streamlining the regulatory handling of medical devices within the EU and ensure more transparency.
Elsewhere in this newsletter you will be able to read “This issue’s portrait” about Martine who is our Product Manager.
Please feel free at any time to reach out to your Area Sales Manager, if you have a wish to discuss future ways of developing business opportunities.
I recommend, that you already now, take the opportunity to speak to your Area Manager for you to coordinate, forecast and specially to place new orders in time.
Please find the New Vitrex Insight with the latest products and updates from Vitrex and the CTI group.
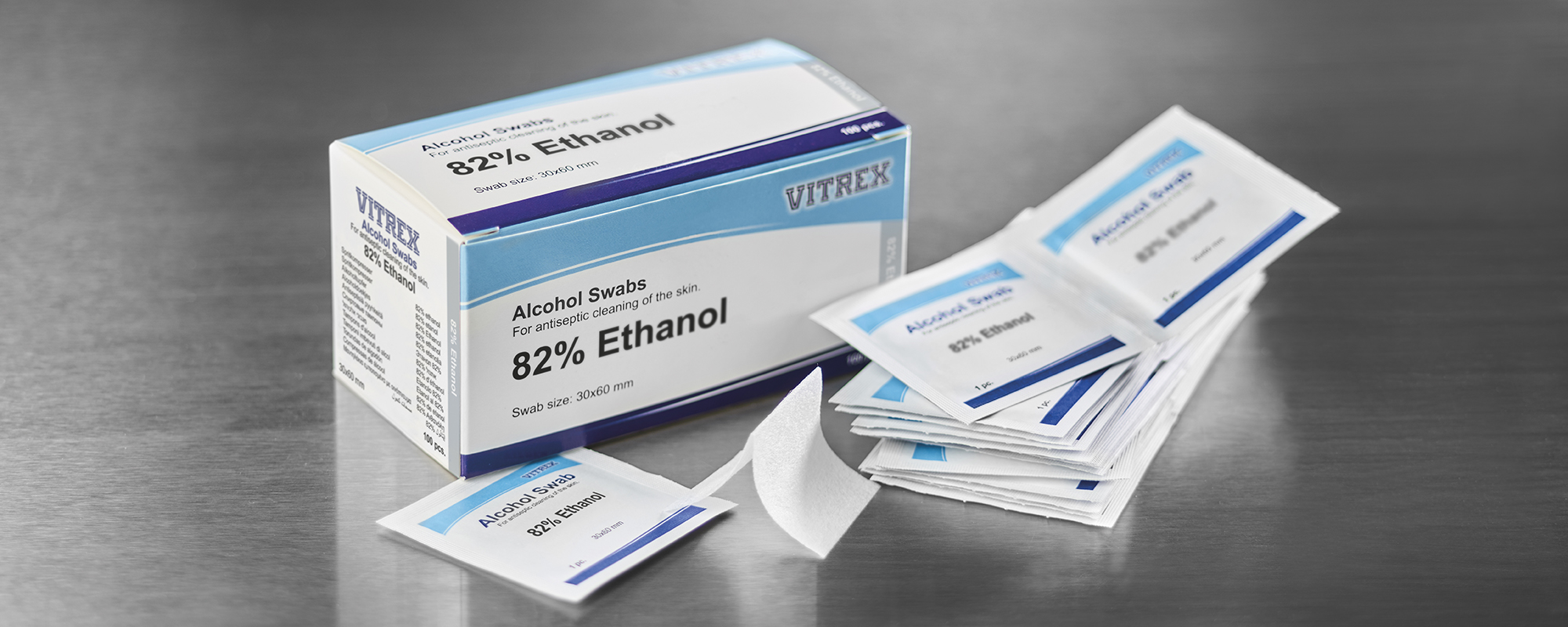
New Alcohol Swabs –
Registered according to the new EU Biocidal Products Regulation
Vitrex Medical has for several years sold Alcohol Swabs with 70% Isopropyl Alcohol. Due to the implementation of the EU Biocidal Products Regulation (528/2012) we were forced to discontinue this product as of 30/6-2023. The reason was that the time/resources/costs associated with re-registration under the new EU Biocidal Products Regulation would heavily collide with a reasonable price setting for this type of product on long-term.
Today we are pleased and proud to inform, that we will during the fourth quarter of 2023 launch two alternative products based on Ethanol – products registered according to the new EU Biocidal Products Regulation. The active substances Ethanol itself is registered with the European Chemicals Agency ECHA as a product type PT 1 (Human hygiene). We are proud because we believe we are the first company in Europe that have managed to achieve this registration of products to be used for human skin disinfection.
The two versions are:
521413 – Disinfection Swabs – Ethanol 82%
522413 – Disinfection Swabs – Ethanol 82%, 0,5% Chlorhexidine.
Exhibitions
Medica 2023
We are happy to inform, that we will be exhibiting at Medica 2023. The exhibition is being held from the 13th to the 16th of November in Düsseldorf, Germany. We are looking forward to meeting with current and potential customers at this year’s Medica. Please come by our stand 3H25 or contact us at vitrex@vitrex.dk to book at meeting with our sales team and get a voucher code. We look forward to presenting our current range as well as our new products. See you soon in Düsseldorf.
This issue’s portrait
Martine Hallestad Bertram has worked for Vitrex Medical as Product Manager for the last 2 years. She has a professional clinical background as a registered Intensive Care Unit Nurse. Many of you might already by now have communicated with Martine when she is assisting in connection with providing regulatory documentation for local registrations or in connection with product trainings facilitated by one of our Area Sales Managers.
Martine lives with her husband and son close to Vitrex and has previously been a serious athlete within the martial art – Taekwondo – having won several championships. Martine holds the Black Belt 😊. In her spare time she enjoys spending time in her families summer cottage in the Northern Part of Zealand.

Martine Hallestad Bertram
Product Manager

Recent Comments